Abstract
Background: MM is a disease of the elderly, with a median age at diagnosis of approximately 65-70 years. Induction chemotherapy followed by high-dose melphalan ASCT is considered the standard of care in younger patients (< 65 years). Historically, transplant eligibility has been determined according to age, based on clinical trials of young MM patients and the potential increased toxicity and mortality in the elderly. However, numerous studies have demonstrated the efficacy and safety in elderly MM patients. Here, we present the largest cohort of elderly MM patients aged ≥ 65 years from Australia and their outcomes based on our multi-centre experience.
Methods: A retrospective case note audit identified 426 MM patients who underwent a single ASCT at the Peter MacCallum Cancer Centre and University Hospital Geelong, Australia, between 2008 and 2016. Patients were analysed based on age at ASCT and divided into the "elderly group" (aged ≥ 65 years) and the "younger group" (aged < 65 years). Patient characteristics including ISS stage, cytogenetics, pre and post transplant response, and engraftment duration were collected. Response assessment included overall response rate (ORR), transplant-related mortality (TRM), progression-free survival (PFS) and overall survival (OS). The Kaplan-Meier method was used to estimate OS and PFS and compared using the log-rank test.
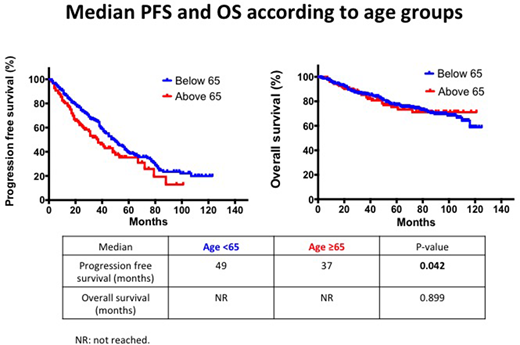
Results: There were 123 patients in the elderly group (median age 67 years; range 65-79,) and 303 the younger group (median age 56 years; range 31-64). Median follow-up time was 74 months. There were no differences in gender and cytogenetic risk status between the 2 groups. The younger group had a higher proportion of ISS stage 3 disease (25% vs 13%, p=0.031), while the elderly group had a higher proportion of ISS stage 1 disease (53% vs 38%, p=0.024). More patients in the younger group received full dose melphalan conditioning (200mg/m2) compared to the elderly group (89% vs 76%) while the elderly group had a higher percentage receiving a reduced dose between <100-180mg/m2 (22% vs 9% younger group). A significant proportion of elderly patients received bortezomib based induction chemotherapy (72% vs 48%, p= <0.001) while the younger cohort had more patients receiving DNA damaging agents (e.g. cyclophosphamide and doxorubicin) likely reflecting the increased availability and tolerability of novel agents in elderly patients. ORR pre (≥PR = 87% vs 88% younger group) and post-ASCT (≥PR = 88% vs 87% younger group) were comparable between the 2 groups. Mean time to neutrophil (11 vs 11 days younger group) and platelet (13 vs 12 days) engraftment were similar and TRM was low (2%) in both groups. The median PFS for the elderly group was 37 months compared to the younger group of 49 months which was statistically significant (p=0.042) while the median OS was not reached in both groups [Figure 1].
Conclusion: The findings of this retrospective study suggest that ASCT is both well tolerated and effective in MM patients aged ≥ 65 years. Therefore despite the increased availability of novel agents, ASCT should still be an essential element of treatment in elderly MM patients who are fit enough for the procedure.
Routledge:BMS: Honoraria; Celgene: Honoraria. Harrison:Janssen-Cilag: Other: Scientific advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal